An update from our Chief Medical Officer:
May ‘the Fourth be with you’! Monday May 4th is a holy day of obligation for Star Wars fans around the world and perhaps an appropriate proclamation for what’s happening in America, as almost half of the United States business community will reopen in some manner this week. I have not yet seen “Rise of Skywalker” but imagine there is some grand finale where the inherent conflict within ‘The Force’, the struggle between good and evil, will play out. In Coronavirus SARS-2 we face a less visible villain than Darth Sidius, yet one no less threatening and I want to reiterate how important it is that we remain vigilant, strong and committed to our mitigation efforts, our ‘rebellion’ against this COVID-19 challenge.
As practices across QualDerm either reopen or enhance services, the pandemic continues to rage across the country and many regions have not yet met the 14-day CDC gating criteria of reduced case prevalence. Daily infection rates remain over 30,000 and last week the US case count eclipsed the 1.1 million mark while deaths topped 60,000, more than the number of military killed in the entire Vietnam War. For anyone who has visited the Vietnam Veterans Memorial in Washington DC, the overwhelming sense of loss and the massive scale of those impacted is unforgettable. COVID-19 is not over yet as 2nd and 3rd waves are expected and are already occurring around the world.
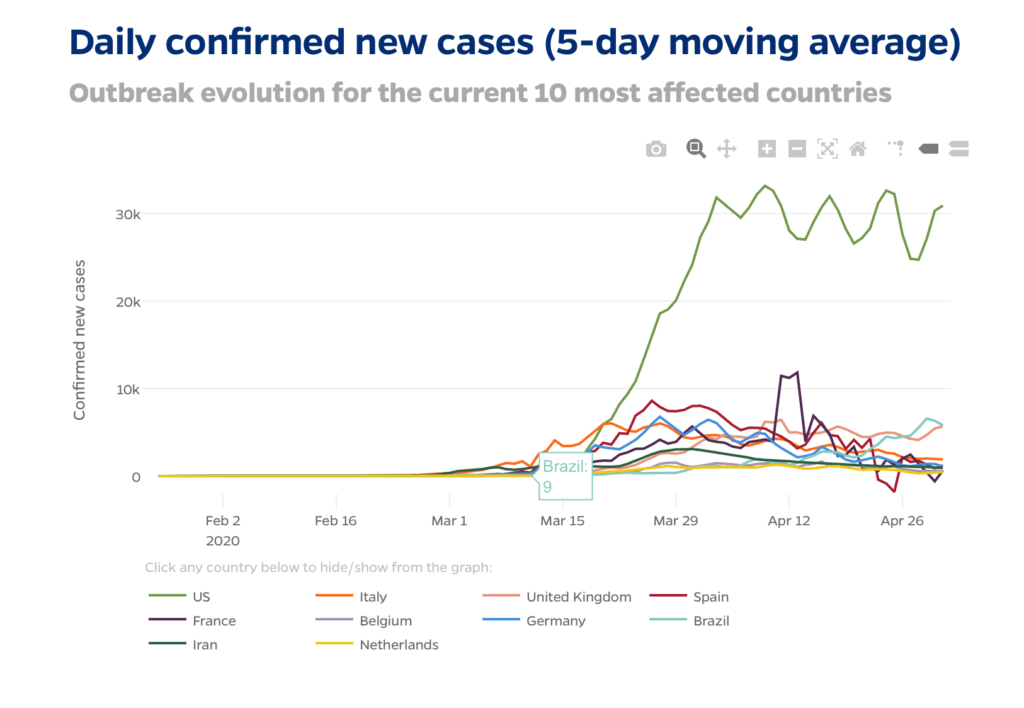
BUT, I choose hope and encourage everyone to also remain optimistic as many positive events have unfolded this week. The overall US Covid Curve has been flattened substantially and generally our healthcare infrastructure and capacity for critical care/ICUs/ventilators has remained sufficient to meet the crisis. We will get through this together!
- Remsdivir is an antiviral medication previously developed for Ebola that shows promise as a treatment option
- Hospitalized, severely ill C-19 patients recovered in the ICU 4 days sooner with Remsdivir (15 vs. 11 days), although case fatality was not reduced
- FDA has authorized emergency use status: patients to be treated earlier in their course with hopes that it can prevent severe deterioration and death
- Operation Warp Speed describes the Administration’s goal for rapid development of a vaccine by January 2021
- Scientists in Oxford England have identified a potential vaccine that could possibly be available by fall 2020; FDA has numerous vaccines in various phases of study and testing with goal of mass production in 2021
- 7 of the roughly 90 vaccine candidates have reached the stage of clinical trials
- US testing capacity has finally become more widespread as the FDA has authorized dozens of manufacturers and labs to produce testing kits; the White House goal is 5M tests daily but the highest daily total to date has been 314,000 (average ~150,000) and experts predict 5M goal unachievable; 5.7 M tests have been conducted to date (more than any other country) but still only 2% of US population
- FDA granted emergency use approval over weekend to Roche Labs for an antibody test reportedly showing 100% sensitivity and 99.8% specificity
- sensitivity = ability for test to identify true positive result (C19 infection)
- specificity = ability for test NOT to identify false results (No C19 infection)
- Unfortunately, the accuracy of many commercial tests remains questionable, especially for antibody tests which seem less reliable
- RNA testing which identifies actual C-19 viral material to determine positivity is more accurate than antibody testing, which relies upon measuring the blood levels and types of antibodies produced by your immune system in response to infection, which is variable based on patient, timing, immune status
- How much and what type of viral material equates to infection is unclear
- Majority of new testing capacity is antibody testing
- New saliva testing available in New Jersey which is easier and less risky
- Interpreting results remains problematic since many questions remain unknown
- Does antibody status equate to protection from infection/re-infection?
- Which antibody(s) and at what blood level is more or less protective?
- How often is retesting required because new infections are possible?
- Which tests kits are more/less accurate and at what cost?
- How available are test kits in local communities and who qualifies for testing if limited quantity? Healthcare workers, factory workers, elderly, immunosuppressed, SNF, prisons, military, 1st responders, etc.?
- US has prioritized efforts to enhance contact tracing capacity so all confirmed C-19 patients can be immediately quarantined along with their close contacts; this effort will allow continued reopening of our economy while containing hot spots and new outbreaks, critical to preventing more surges of infection
- The supply chain for personal protective equipment (PPE) like masks, gloves, gowns, face shields, ventilators has improved; costs remain high but generally supplies are now more available
- Some state medical societies like NCMS have developed discrete PPE supply chains to enhance small practices’ ability to procure this essential equipment
- More studies have identified additional signs and symptoms of COVID-19 and research is learning how the virus attacks the body and strategies to combat these effects
- In addition to cough, shortness of breath/difficulty breathing, and fever, CDC now considers these features also consistent with C-19 infection
- Chills
- Repeated shaking with chills
- Headache
- Muscle pain (body aches)
- Sore throat
- New loss of taste or smell
- Dermatologists have identified several skin conditions in C-19 patients
- “COVID Toes” – painful red-purple tender lesions on toes/fingers
- AAD publishing a report this week of first 300 C-19 patients in national registry for skin conditions associated with infection
- In addition to cough, shortness of breath/difficulty breathing, and fever, CDC now considers these features also consistent with C-19 infection
- FDA granted emergency use approval over weekend to Roche Labs for an antibody test reportedly showing 100% sensitivity and 99.8% specificity
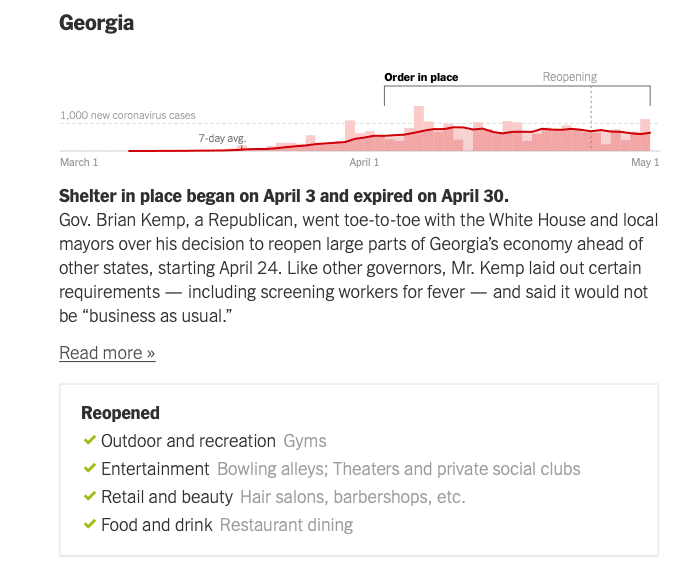
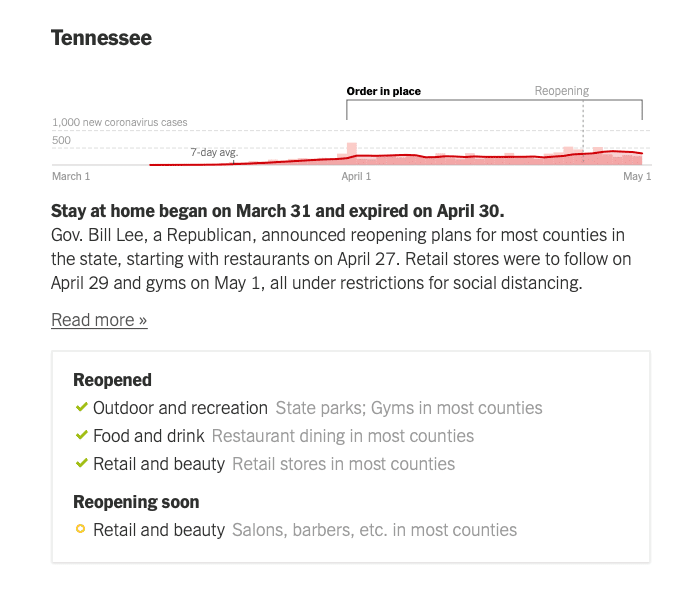
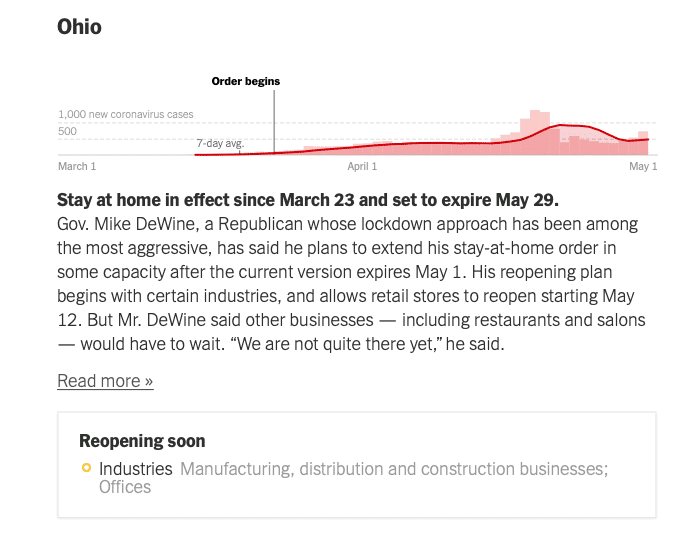
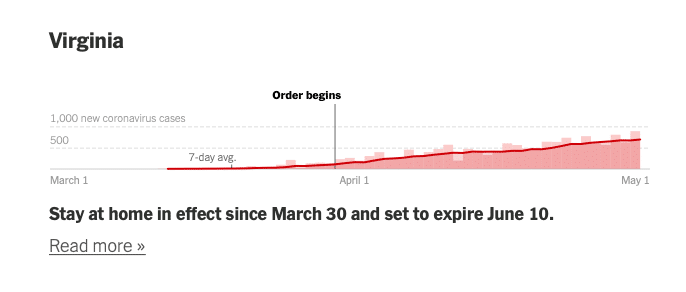
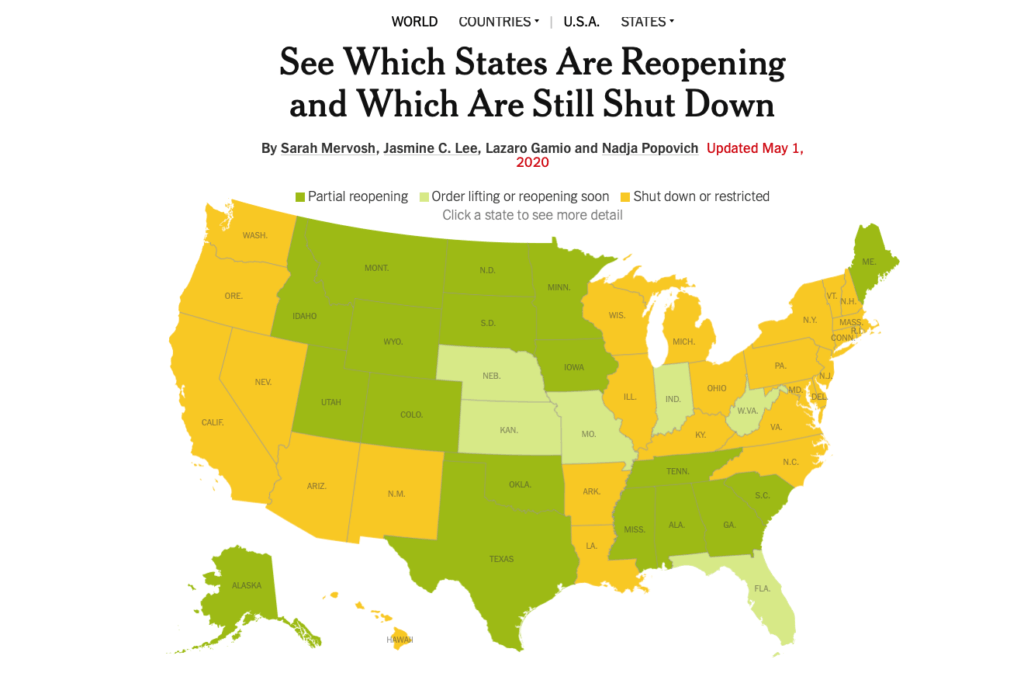
Here are some diagrams showing local and regional trends. Fortunately, many of our QualDerm practice sites serve more rural or suburban communities where the COIVID-19 impact has been less severe, allowing for ongoing urgent care and opportunities to more quickly reopen and address postponed, rescheduled and elective visits. I want to reiterate how important it is to follow your local, state and federal orders and CDC guidelines for social distancing behaviors. Each state Joint Operations Committee (JOC) comprised of your physician and operations leadership will consider local conditions and determine how and when to open offices.
National polls repeatedly show that public trust in healthcare providers is extremely high, as it should be! Let’s all continue to maintain this vital trust by not compromising our values. Please walk the walk, talk the talk, wear your masks to the store, don’t congregate together or have a dinner party, wash your hands and stay home if sick. Our QDP Reopening Plans will only be successful if we follow the checklist diligently. Our exhaustive checklist has been developed with inputs from every facet of the company and is a true testament to all of you and your commitment to our mission. Teammates will keep working remotely from home as much as possible and offer TeleDermatology services for vulnerable patients and to reduce clinic volumes. Our TeleDerm Task Force is optimistic about many enhanced features and better usability from our EMR vendors (both NexTech and EMA) who are updating products now.
I continue to respond to questions from QualDerm team members regarding PPE, Covid testing, reopening policies, triage and screening. I remain committed to providing as accurate, factual, and updated information as possible and appreciate the ongoing dialogue with all of you.
Thanks for keeping the Qual in QualDerm!
Sincerely,
John Albertini, MD